
思摩尔:毛利下滑,口味政策下的需求仍待确认

以下为思摩尔 2022 年中期财报电话会纪要:
管理层发言
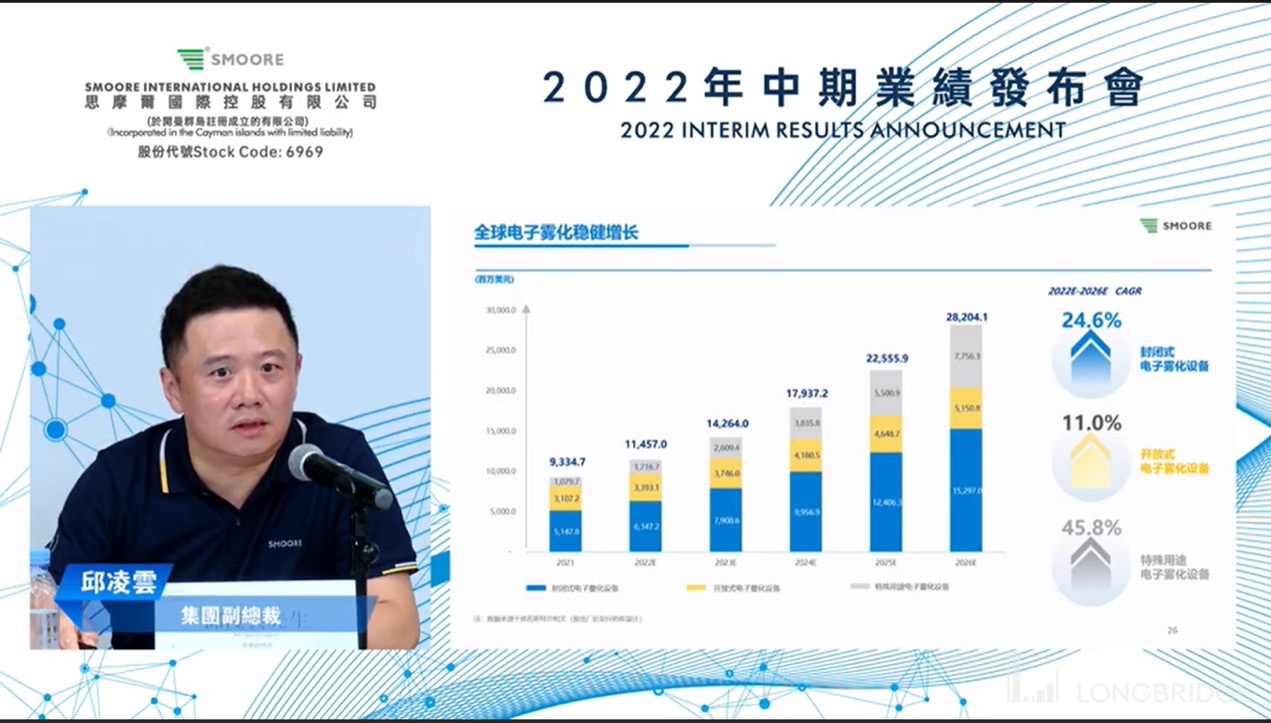
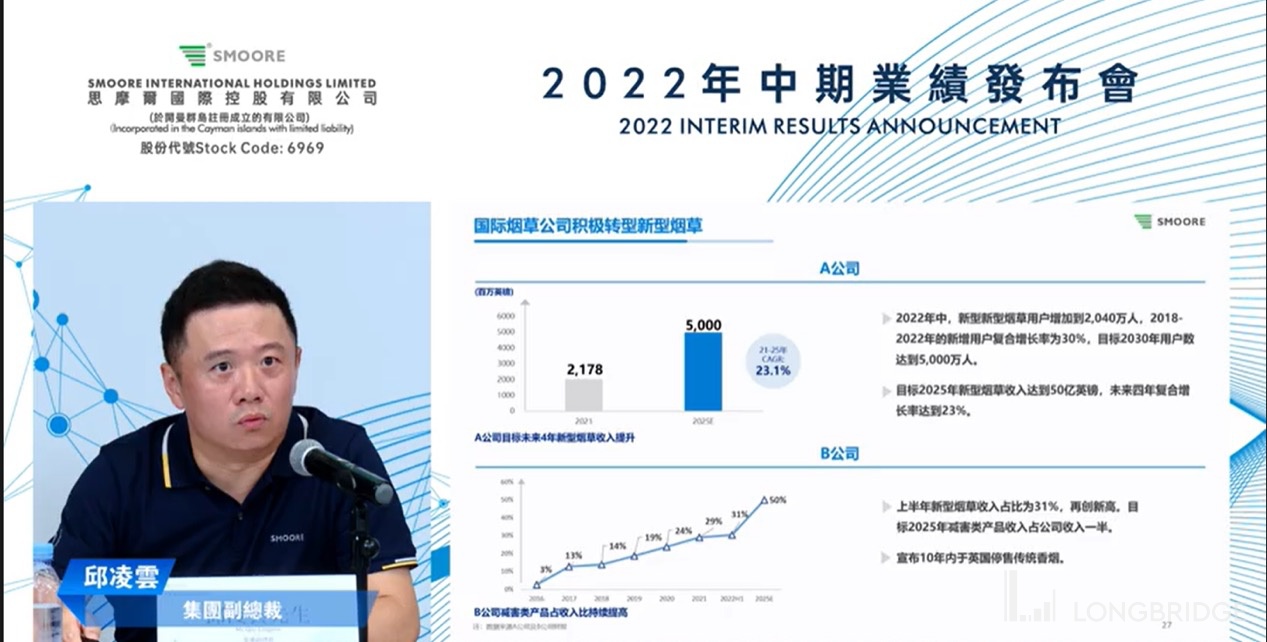
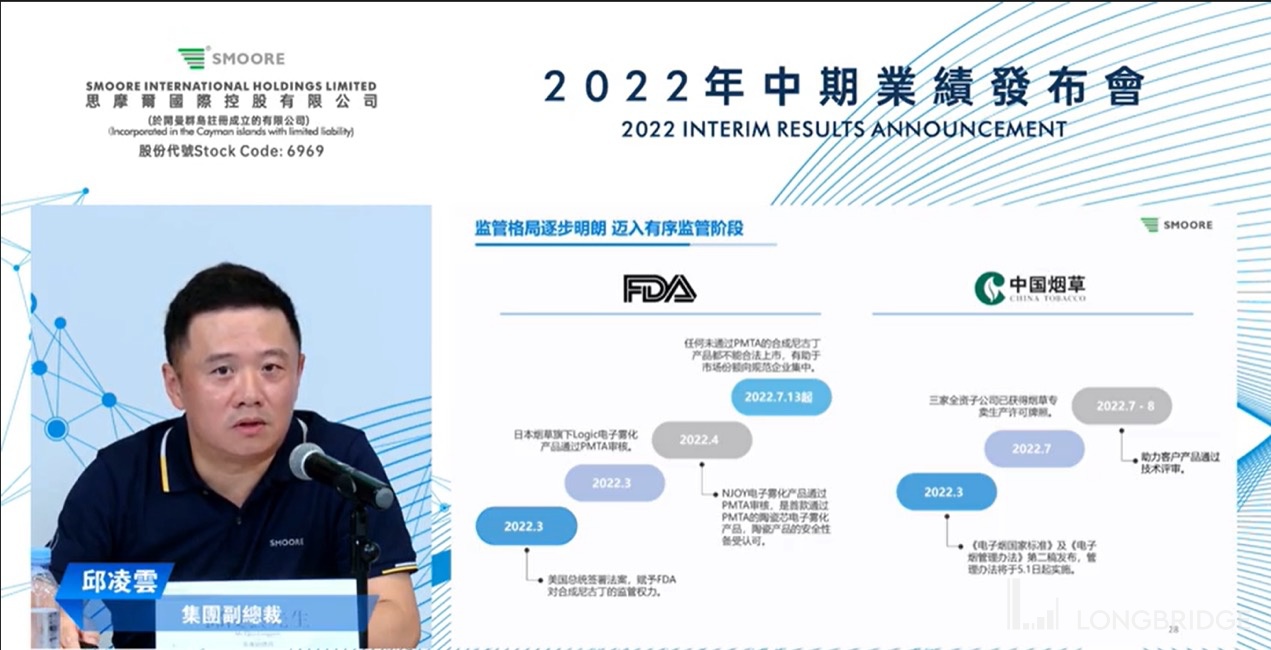
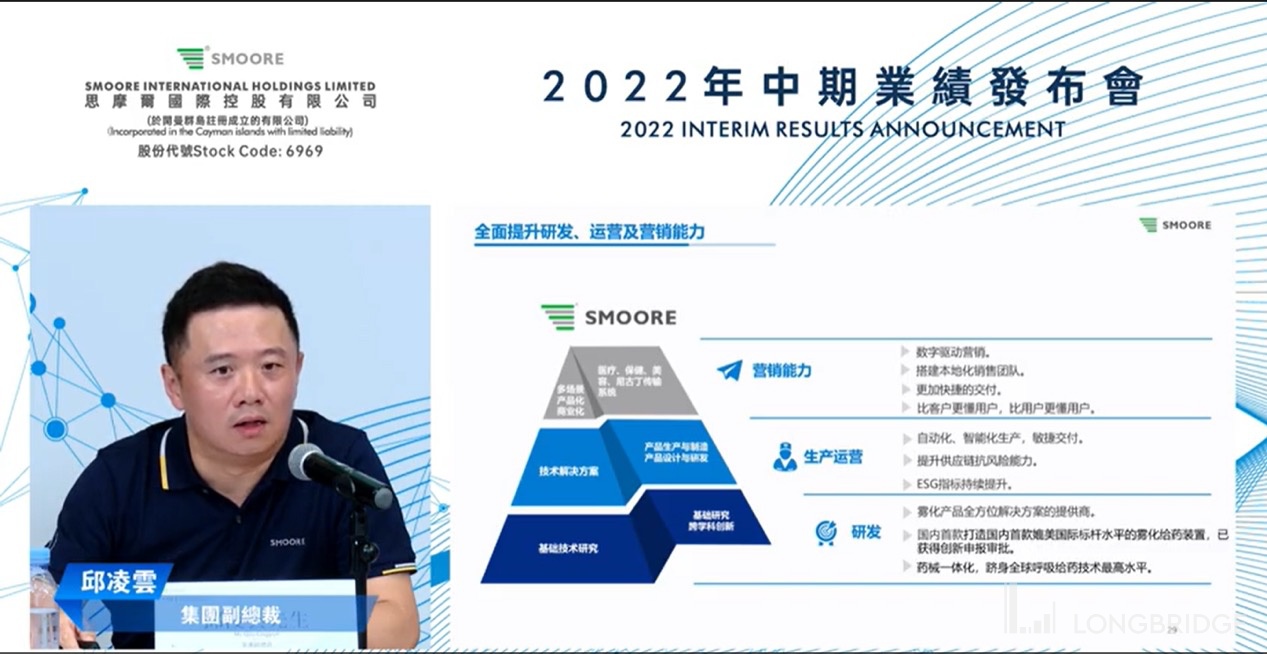
分析师问答
Q:公司上半年研发费用率达到 11%,研发投入明显加大,能否分享下未来的研发成果
A:公司上半年的研发投入的具体实施情况和落地情况符合全年的整体布局。上半年研发团队的规模在扩充,品类研发有序进展,增加了 7 个研究院,覆盖到了雾化医疗和雾化材料领域。
Q:半年度业绩公告提到说下半年收入会比上半年有一个明显提升,原因是什么
A:1)一次性产品是在今年二季度开始环比增长,趋势还会继续。2)欧洲整体增长不错,中国下半年,我们认为第四季度仍有一定挑战,我们希望通过海外增长弥补国内第四季度收入的下降,这是我们对市场的判断。
Q:医疗美容业务什么时候开始有收入贡献
A:1)今年下半年两个产品品类都会有一些交付,医疗美容的产品在 9 月份开始会在用户端进行小规模的试销。2)国内首款雾化给药装置已经完成了创新认证,按照规划,在年内能完成整个产品的注册,进入销售状态。3)我们在美国的医疗研究院、美国医疗子公司的整体运营团队的组建等进展很顺利。
Q:对于年内和长期毛利率的展望
A:取决于两个因素:1)产品差异化和技术进步;2)和客户双方的谈判以及利益分配。
长期来看,我们不断加大研发投入打造差异化产品,希望通过创新,一方面帮助客户增加收入,另一方面让我们获得比较满意的毛利率。
短期内,1)不同产品组合的变化会对我们的毛利率有一定影响。一次性产品目前量不多,现在的毛利率水平还比较低,随着量上来以及对供应链的不断优化,希望把毛利率提升,但是未必能达到现在换袋式的毛利率水平。2)市场上的医疗类产品很多都是被欧美公司独家供应,毛利率非常高,我们希望打破垄断,或者在国内市场,为消费者提供可以负担的医疗产品来提高我们的毛利率。
Q:公司大客户布局了一次性电子烟产品,一次性产品出货较 21 年有两倍以上的增长,未来一次性产品的出货量和毛利率展望
A:上半年,一次性产品取得了 3 亿左右的营收,我们的大客户也接受了我们的一次性产品,也在欧洲、英国取得了非常好的市场业绩。下半年订单规模会更大,销售规模也会比上半年大。
Q:我们和大客户英美烟草的产品定价的同比表现情况
A:我们上半年全面推行了自动化,降低了人工成本,同时支持客户在全球化上做更好的推广,扩大他的市场份额。所以产品定价的同比主要表现在自动化上。
Q:在 PMTA 框架下,公司与英美烟草的关系有没有什么变化
A:目前是非常健康,以后也是越来越加深合作的关系。1)我们的自动化,是基于两家公司对产品未来的长期看好以及我们在生产工艺品质控制达成一系列共识的基础上,和英美烟草推出了全球最高速的烟袋生产线,是双方合作达到的一个新高度。2)我们今年上半年推出的产品快速占据了英国第二位的品牌地位,这也说明我们和英美烟草等大客户的合作会越来越紧密。
Q:NJOY 和 Logic 两大客户通过 PMTA 之后,整个经营趋势是否出现明显好转
A:两家公司通过 PMTA 以后,订单有逐步上升的趋势,客户明显增强了他们对美国市场的信心。
Q:美国市场那边收入同比下降的原因
A:两个原因,1)英美烟草等大客户的销量并没有下降,因为自动化,我们的销售价格有下浮,所以上半年来自美国市场的收入有所下降。2)去年我们的大客户的物流方式从空运变为海运,去年多了整整一个月的出货量来保证安全库存。今年上半年,全部导入海运运输,有效控制去年多的一个月的出货量。今年上半年,客户考虑到库存减少了一定的出货量,以控制它在美国整体的库存。
Q:美国市场的薄荷口味烟被否决的原因,未来的前景
A:FDA 否决薄荷烟的产品,在官方网站的新闻稿有解释。两个原因:1)一些有害物质、化学物质超标;2)数据前后不一致。这是官方否决的解释,否决的不仅是薄荷味这一种。
未来走向:不好妄加猜测。1)我们认为还是要遵循第一性原理,消费者使用尼古丁的电子烟是因为对尼古丁的需求,口味不是主要因素,美国对水果口味发布禁令后,剩余口味快速反弹也验证了这一点。2)欧洲、美国在限制薄荷口味的传统卷烟,这个大趋势有利于传统烟民转向新型烟草制品。
Q:公司欧洲及其他地区收入增长比较明显的原因
A:两点:1)欧洲大客户的贡献,换袋式产品从第二名到第一名,其次,他新进入市场的一次性产品取得了一个非常重要的市场地位。2)上半年,开放式的 APV 产品表现突出,源于核心技术发布带来的差异化的产品表现以及 APV 在欧洲市场的本地化团队建设。
本文的风险披露与声明:海豚投研免责声明及一般披露
本文版权归属原作者/机构所有。
当前内容仅代表作者观点,与本平台立场无关。内容仅供投资者参考,亦不构成任何投资建议。如对本平台提供的内容服务有任何疑问或建议,请联系我们。



