
Pfizer's new cancer drug Mevrometostat has initiated Phase 3 clinical trials in China, showing promising anti-cancer activity in preliminary studies

Pfizer has initiated an international multicenter Phase 3 clinical trial (MEVPRO-1) to evaluate the efficacy and safety of its new drug mevrometostat in combination with enzalutamide for patients with metastatic castration-resistant prostate cancer. The study plans to recruit 600 patients globally, including 90 from China. Mevrometostat is an EZH2 inhibitor designed to reduce cancer cell proliferation by blocking abnormal gene expression in cancer cells and inducing cell death
According to the financial news app Zhitong Finance, Pfizer (PFE.US) has recently initiated an international multicenter Phase 3 clinical trial (MEVPRO-1) in China, aiming to evaluate the efficacy and safety of PF-06821497 in combination with enzalutamide compared to enzalutamide or docetaxel in patients with metastatic castration-resistant prostate cancer who have previously received abiraterone acetate treatment. The study plans to enroll 600 participants internationally, including 90 in China.
Public information indicates that PF-06821497 (mevrometostat) is an investigational EZH2 inhibitor. According to Pfizer's disclosure during its Oncology Innovation Day in March this year, the company is expected to launch over 8 significant therapies in oncology by 2030, including this EZH2 inhibitor.
EZH2 has become one of the forefront targets in cancer treatment. EZH2 is a histone methyltransferase, and its abnormal expression is associated with the progression and poor prognosis of various cancers, including prostate, breast, lung, and hematologic malignancies. Studies have shown that overexpression of EZH2 can promote cancer initiation and progression through various mechanisms, including silencing tumor suppressor gene expression and promoting genes involved in cell proliferation and survival. Additionally, EZH2 has been shown to promote cancer metastasis by regulating gene expression involved in epithelial-mesenchymal transition.
Mevrometostat is a Pfizer-developed inhibitor targeting EZH2, which can block the abnormal gene expression in cancer cells driven by EZH2 overexpression, leading to reduced cancer cell proliferation, induced cell death, and inhibition of cancer cell migration and invasion, ultimately achieving the goal of cancer treatment.
Based on clinical data disclosed by Pfizer in March this year at the Oncology Innovation Day, the combination therapy of mevrometostat and enzalutamide (Xtandi) showed promising anti-cancer activity in a Phase 1 clinical trial for patients with metastatic castration-resistant prostate cancer (mCRPC). Specifically, in patients previously treated with abiraterone, the median radiographic progression-free survival (rPFS) with mevrometostat+enzalutamide treatment was 17.1 months, a significant improvement compared to historical data (rPFS of 4.8 months).
According to ClinicalTrials website, Pfizer initiated a Phase 3 clinical trial (MEVPRO-1 study) in the United States in August this year targeting mCRPC patients previously treated with abiraterone. This also marks Pfizer's clinical research initiation in China
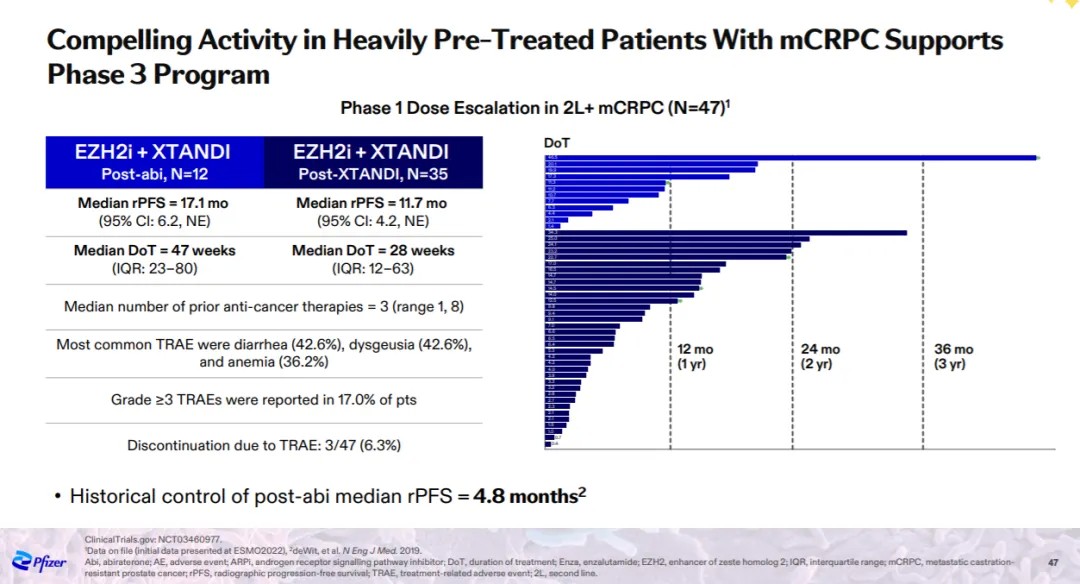
Clinical trial results of Mevrometostat (Image source: Pfizer official website)
In addition, Pfizer has recently initiated another Phase 3 study, MEVPRO-2, to evaluate mevrometostat + enzalutamide for metastatic castration-resistant prostate cancer male patients who have not been treated with abiraterone. The Investigational New Drug (IND) application for this indication has also been approved in China and is expected to start in China soon.
Studies have shown that EZH2 inhibitors have demonstrated potential in treating various types of cancers, including B-cell lymphomas, solid tumors, and certain other hematologic malignancies. For example, tazemetostat has been approved for treating some types of hematologic tumors. Meanwhile, there are multiple EZH2 inhibitors under development globally to treat various types of tumors

