
The global FIC/BIC innovation matrix leads the endocrine track, analyzing the core logic of VISEN PHARMA's value growth

維昇藥業於 3 月 13 日開啓招股,預計 3 月 21 日在港交所上市,全球發售 990 萬股,招股價 68.44 港元至 75.28 港元。此次 IPO 引入 5 名基石投資者,投資者需關注公司創新產品管線的商業價值。維昇藥業專注於內分泌領域,採用 “大引擎 + 小引擎” 模式,滿足市場需求。
2025 年以來,港股恒指大盤兩個月內拉昇 20%,本質反映出國際資本對中國資產的定價邏輯發生結構性轉變,由此帶來整體市場的估值上修期。對於兼具稀缺性和高確定性的維昇藥業而言,此時登陸資本市場正逢其時。
智通財經 APP 瞭解到,維昇藥業已於 3 月 13 日開啓招股,預計將於 3 月 21 日在港交所上市。招股書顯示,公司擬全球發售 990 萬股,其中香港發售股份數目為 99 萬股,國際發售股份數目為 891 萬股;招股價為 68.44 港元/股至 75.28 港元/股。
公司此次 IPO 招股引入 5 名基石投資者,包括安科生物、蘇州工業園區產業投資基金、Vivo Capital、藥明生物、Reynold Lemkins。
IPO 行至招股階段,也就意味着維昇藥業打新通道的開啓。在港股市場打新一家創新藥公司,投資者需要從公司潛在增長空間和創新研發實力兩大關鍵要素出發,把握公司創新產品管線背後的超預期商業價值,而這同樣也是未來投資者長期配置維昇藥業的核心邏輯。
大小引擎雙驅發力,合力打開估值天花板
與目前多數港股創新藥企扎堆腫瘤或自身免疫等熱門賽道不同,維昇藥業頗具特色地選擇了擁有一定技術門檻,但存在龐大未滿足治療需求的內分泌賽道作為其主打管線方向,並以 “大引擎 + 小引擎” 模式構建商業化版圖,覆蓋從常見病到罕見病的完整內分泌治療場景。
而這顯然基於維昇藥業對臨牀需求的深刻洞察。
據弗若斯特沙利文數據,中國的非糖尿病類內分泌藥物市場規模呈現出強勁增長,由 2018 年的人民幣 117 億元增至 2023 年的人民幣 208 億元,年複合增長率為 12.2%;預計到 2030 年將進一步增至人民幣 742 億元,自 2023 年至 2030 年的年複合增長率為 19.9%。
不過,與此龐大增量市場相對的,卻是國內同賽道創新藥企寥寥無幾。其根本原因在於內分泌治療賽道存在較高的技術准入門檻。
以兒童生長激素缺乏症 (PGHD) 為例,作為人們常説的矮小症的一種,該適應症是由於腦垂體生長激素分泌功能障礙所引起,兒童患病率約為 1/5000。
從治療手段來看,短效重組人生長激素目前是兒童生長激素缺乏症最常見的治療方案。但其治療方案需每日注射,嚴重影響患兒的日常生活,治療依從性差。另有研究表明,患兒使用生長激素日製劑漏針現象普遍存在,而一週若漏打 1-2 次將會明顯降低治療效果。
因此,使用注射頻率更低、患者依從性更高的長效生長激素 (LAGH),已成為生長激素市場發展的必然趨勢。預測數據顯示,全球生長激素市場 2030 年有望達 48 億美元,中國市場佔比超 34%。而維昇藥業依託全球首款被證實優效於生長激素日製劑的長效生長激素——隆培促生長素 (lonapegsomatropin,TransCon hGH) 將搶佔這一戰略高點。
與同類長效方案相比,維昇藥業隆培促生長素採用的是 TransCon(Transient Conjugation,暫時連接) 專利技術為前藥平台。與短效方案相比,除了每週注射一次外,隆培促生長素也顯示出了優秀的療效數據,根據已上市 LAGH 產品的公開數據,隆培促生長素是目前唯一經頭對頭臨牀試驗證實比生長激素日製劑療效更好的長效產品,顯示出了明確的 BIC(Best-in-Class) 屬性,預計上市後將佔據中國 LAGH 市場的領先地位。
目前,隆培促生長素在歐美獲批。值得一提的是,隆培促生長素 (海外商品名:SKYTROFA),作為美國首個用於治療兒童生長激素缺乏症的長效生長激素,在 2023 年第四季度問鼎美國生長激素領先品牌。據 Ascendis Pharma 財報顯示,2024 年隆培促生長素海外的銷售額為 1.97 億歐元。
除了隆培促生長素這個商業化 “大引擎”,作為維昇藥業中長期商業化 “小引擎”,帕羅培特立帕肽 (palopegteriparatide,TransCon PTH) 同樣值得關注。
作為全球 FIC(First-in-Class) 品種,帕羅培特立帕肽是維昇在研用於成人慢性甲狀旁腺功能減退症 (甲旁減,HP) 的激素替代治療藥物,旨在通過每天一次皮下注射,維持每天 24 小時內甲狀旁腺激素的生理水平。
甲旁減是最後一種尚未實現激素替代治療的內分泌激素缺乏性疾病。從市場前景來看,該疾病存在潛在 40 萬患者羣體,並有強烈治療需求,需要終身持續用藥治療。
帕羅培特立帕肽作為全球首個甲旁減激素替代療法,或將顛覆傳統治療方案及治療標準,並促進甲旁減治療指南的調整。目前該產品已先後在歐盟、英國及美國獲批上市。
2023 年 1 月,帕羅培特立帕肽中國 3 期關鍵試驗雙盲期完成,達到主要複合終點,進入開放性擴展階段。而在維昇高效的臨牀推進下,帕羅培特立帕肽在國內的商業化進程正持續加速,未來獲批上市後,有望成為國內首個針對甲旁減疾病的激素替代療法,助力患者擺脱傳統治療困境,同時也將持續增厚公司收入水平。
從商業化構建角度來看,維昇藥業已圍繞隆培促生長素和帕羅培特立帕肽兩大關鍵品種,構建起一套未來支撐公司中長期穩健增長的商業化 “大小引擎”。
公司商業價值增長確定性將持續提升,合力打開公司的估值天花板。
高效開發驅動內在價值不斷提升
雖然成立時間不長,但維昇藥業能在 6 年內完成從研發到產品上市申報的全流程突破,主要得益於公司核心團隊均擁有跨國藥企 20 年以上經驗,涵蓋藥物發現、臨牀開發與商業化全週期,充分展現出其 “高效率、強執行力” 的 Biotech 特質。
目前維昇藥業已形成了由 3 款內分泌管線——新型長效生長激素 (隆培促生長素)、軟骨發育不全治療藥物 (那韋培肽) 以及甲旁減治療藥物 (帕羅培特立帕肽) 構成的內分泌產品管線。維昇藥業管線的 3 款內分泌治療藥物在中國的臨牀試驗中均達到臨牀終點,與海外臨牀試驗結果一致,TransCon 技術平台也因此在中國得到了有力驗證。TransCon 技術平台通過 “暫時連接” 分子設計,可以延長藥物半衰期至數週,同時保留原型藥物的生物活性。
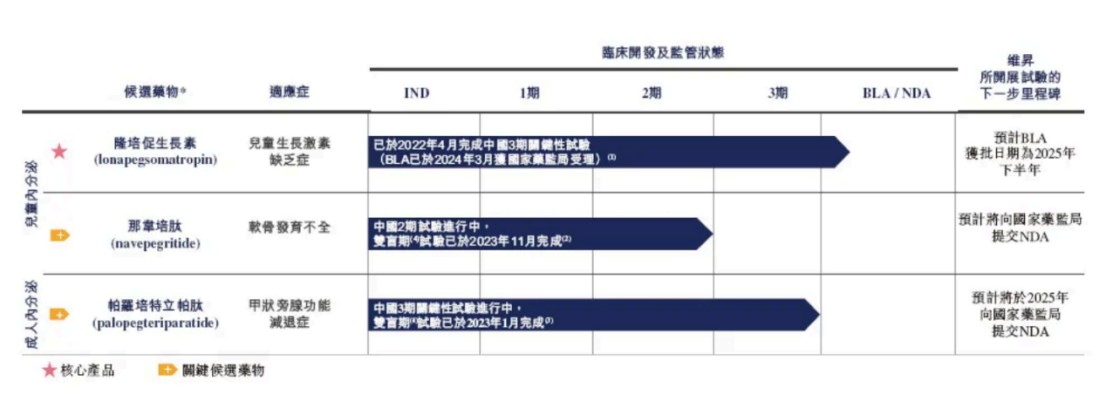
除大小引擎之外,維昇藥業的另一款關鍵候選藥物同樣也是同類首創 (FIC)。針對罕見病軟骨發育不全 (ACH) 的管線產品那韋培肽,當前中國 2 期臨牀試驗完成,達到主要終點。據沙利文數據,2023 年中國軟骨發育不全的患病人數為 5.12 萬例。
值得一提的是,維昇在成立之初就與中國罕見病聯盟和北京大學臨牀研究所等國內知名組織和學術機構達成戰略合作,通過整合產學研資源,開展相關流利病學調查研究,推動對中國相關部門對內分泌疾病及罕見病流行病學現狀的全面瞭解與掌握,助力中國軟骨發育不全 (ACH) 及甲狀旁腺功能減退症 (HP) 的疾病的防治與保障工作,充分體現了維昇以患者為先的原則和社會責任感。
綜上所述,維昇藥業在細分賽道、技術壁壘和高效研發三方面均符合市場對一家優質的創新藥公司打新的價值判斷。基於這些優點,早在 B 輪融資時,公司便被紅杉中國基金選中領投,並獲奧博資本,夏爾巴投資等眾多知名機構跟投,體現出機構投資者對維昇藥業投資價值的高度認可。
在港股 Biotech 研發同質化的背景下,追求在內分泌賽道精耕細作的維昇藥業順利上市後,有望憑藉差異化賽道佈局、核心技術壁壘與高效執行能力,成為板塊內稀缺的 “高增長、高壁壘、高確定性” 標的,不斷釋放自身內在價值。

